
Send any comments to the maintainer Roger Caffin
There have been a lot of myths and misunderstandings in this area. Let's start by making some facts clear. Further explanations are given below.
I have had some long email discussions with a couple of experts in this area. They have added to my education, for which I am grateful. However, I accept full responsibility for what is written here.
| Contents |
|---|
|
I distinguished between 'mixture' and 'solution' in the stoves pages. In fact the situation is more complex, but before we get into the details we need to spell out some basic science background. That way I can keep the subsequent explanations fairly short and simple.
I distinguished between 'mixture' and 'solution' in the stoves pages. In fact the situation is rendered more complex by two factors. The first problem is that there is not a single definition for the word 'mixture' - or maybe we should turn that around and say that there is no single accepted term for a mix of (say) butane and propane. Some call it a solution; others call it a mixture. This can depend on which industry you are in. So you can call it either.
Now let's think about what happens when we mix butane and propane liquids. They will mix quite happily as both are non-polar. With all that jostling around, many of the bonds between butane molecules will be broken and replaced by bonds between butane and propane molecules. The bonding energies are so similar the molecules don't really care. The resulting liquid will look similar to pure liquid butane for instance. It fits the definition of an 'ideal solution' just as nicely as it conforms to 'mixture'.
Paraphrasing one of my corrspondants (Jim) now: propane and butane interact via van der Waals forces, but they interact nearly equally with one another as with themselves. For that reason the mixture is called an 'ideal solution', and why it conforms well to Raoult's Law. [This also applies to propane/isobutane mixtures.] In contrast, an ethanol-water mixture deviates positively from ideality and has a higher vapour pressure than Raoult's Law predicts. This is because the interaction energies between water molecules, between ethanol molecules, and between water and ethanol molecules, all differ.
If you want more science, I suggest a good text book.
Boiling happens when the total vapour pressure (VP) of the liquid exceeds the surrounding air pressure. The VP of water reaches 'one atmosphere' at 100 C, and that's where it boils. Well, of course, if you are way up a mountain where the air pressure is lower than 'one atmosphere', the water will reach that pressure at a lower temperature and boil below 100 C. This happens. Exactly the same applies to the fuels we use. They 'boil' when their VP reachs the surrounding air pressure.
But evaporation will still happen below the 'boiling point': think of hot water steaming away. In fact a liquid is losing vapour at all temperatures - but the rate of loss will vary. Note that as a volume of liquid loses vapour it is losing the most energetic molecules, so the temperature of the remaining liquid will fall. Any user of gas stoves will know how cold the bottom of the canister gets when the stove has been running for a while.
Butane boils at -0.5 C, while propane boils at -42 C. The difference in boiling point (BP) is due to the difference in masses of the molecules and the different intermolecular forces between the molecules. This is all determined by the size and shape of the molecules. The detailed chemical construction of the molecule plays a significant part in this, but we will ignore that for the present. With these as the extremes, the 'boiling point' of a mix of these two liquids will of course be somewhere in between.
What is the lowest temperature you can get vapour from a canister? When the temperature of the liquids falls below its current boiling point the pressure inside the canister will no longer drive vapour out. That will depend on the mixture of fuels in the canister, and is discussed further on.

So far we have mostly considered ordinary butane, which boils at -0.5 C. Some manufacturers, including MSR and Kovea, offer canisters which contain isobutane instead of butane. Isobutane boils at -12 C. What is the difference, and what difference does this make?
The difference between butane and isobutane lies in the arrangement of the carbon atoms. There is only one way to string three carbon atoms together: in a simple chain of three. But butane has a fourth atom: where does it go? If it connects to the end of the chain you have butane (or n-butane). If it connects to the atom in the middle the chain is no longer a simple straight one, and you have isobutane. For those with pencil and paper and an enquiring mind, you can now try to work out how many variations there might be for 5 or 6 or more carbon atoms. Yeah - messy! and don't forget that you can make a ring with 6 carbons: a benzene ring.
The technical answer to what difference this makes is really 'not much': it's all a matter of degree. (Sorry about the pun!) The energy of the bond between the molecules changes a little because the shape of the molecule has changed, and this is responsible for the shift in the nominal BP. But the mixture still behaves like an ideal solution and obeys Raoults Law. The big difference for us comes when we consider the lower limits of boiling.
What happens if I hold a canister of 80% butane / 20 % propane mix at -5 C? Does all the propane boil off leaving all the butane? No. You are quite close to the boiling point of butane and it will still be giving off lots of vapour. Think of a pot of very hot water - still giving off steam. The liquid butane would still be giving off butane vapour at -5 C, just at a lower rate.
What happens here with a mixture is that the vapour pressure (VP) in the canister will be a combination of the vapour pressures of pure butane and propane. If you have 80% butane in the liquid, the partial pressure of the butane vapour will be 80% of the nominal VP - what it would be for pure butane. So the partial pressure of the propane will be 20% of the nominal VP. If we know what VP curves are for the pure liquids, we can calculate what the VP curve for the mixture would be.
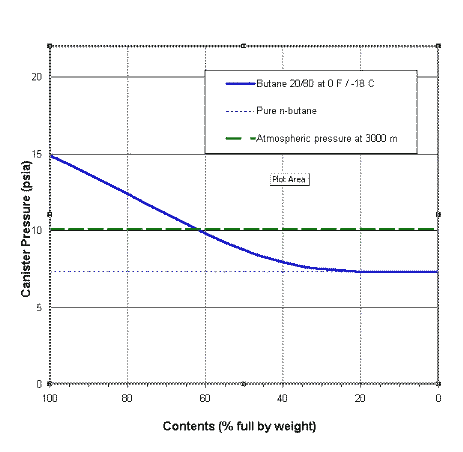
Consider a canister driving an upright gas stove with a gas feed. What we find is that we have a changing situation. The liquids are evaporating: butane and propane are boiling off at different rates, with propane boiling off faster. So the amount of butane and propane left behind will change with time, at different rates, and this will change the mixture left behind and the VP of the mixture as well. If you plot out the pressure inside a gas canister as a function of canister contents you get something like the diagram to the right. This is for a canister which started with 80% butane and 20% propane and is sitting at the quite uncomfortable temperature of -18 C. My thanks to one of my readers for this graph.
The solid blue curve shows the actual pressure as a function of the amount of mixture left inside the canister. This pressure falls as the amount of mixture left in the canister falls. Note especially that the ratio of gases in this mix is changing as gas is drawn off: the amount of propane left is falling.
The faint blue dotted line (joining the blue curve at the right) shows what the gas pressure would be if the liquid in the canister was pure butane: it is a constant of course. It may actually be misleading to draw it as a line across the graph, but never mind.
The dashed green line represents atmospheric pressure at 3000 m. OK, we don't get that high in Australia, but never mind. It too is really independent of the horizontal axis.

Now, what can we tell from this graph? We can see that initially the canister pressure (blue curve) will be above the surrounding atmospheric pressure (green dashes), so gas will come out when you open the valve: a mix of butane and propane. So the canister will be losing both propane and butane. But the propane is 'boiling', while the butane is merely 'steaming', so the propane is coming off at a faster rate than the butane. This means that the concentration of propane remaining in the liquid will fall fairly fast. And as the concentration of propane falls, so does the total vapour pressure. Eventually, as you can see, the VP of the fuel (blue curve) falls below one atmosphere (dashed green line), at which point the canister will stop giving off gas. The stove goes out - even though the canister may still be half full (or more). This has happened to many people in the snow.
Not considered here is the fact that as the fuel evaporates inside the canister the remaining liquid fuel is losing energy and getting colder. This imply makes the sitaution even worse of course: the pressure inside the canister will be even lower. You can see what can happen in the marvelous picture here of an iced up Snow peak stove. The stove is burning all right, but in a little while it will go out when all the propane has been used up.
 |
 |
Here we have graphs showing two other aspects of the physics. The one on the left shows the concentration of butane or isobutane in the vapour at different temperatures and for different concentrations of propane (10%, 20% and 30%) in the liquid in the tank. You can clearly see that the concentration of butane (red lines) does not fall all that much as the temperature crashes downwards (more on that in a minute), although the amount of propane in the liquid certainly does have some effect. The butane vapour (or isobutane vapour) will still be there in the gas. What this graph does show is that when you change from butane to isobutane the concentration of the (iso)butane does rise a bit. But in all cases, the fraction of the gas which is (iso)butane is significant. However, this is not the whole story of course, and the graph to the right shows why.
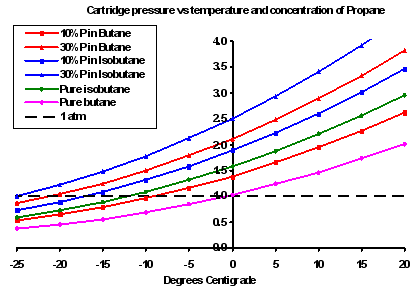
The graph to the right really is the bottom line for many people. It shows the pressure in the canister as a function of the temperature of the liquid, the chemistry (butane or isobutane) and the propane fraction (10% or 30%). The vertical axis is the canister pressure in atmospheres; the black dashed line represents one atmosphere. As the temperature falls so the internal canister pressure falls, until at some rather chilly value the internal prressure is equal to or less than the external pressure. At this point the gas stops coming out of the canister, and the stove stops running.
What is clearly evident here is the significant effect caused by changes in propane percentage and whether the canister has butane or isobutane. You had better also note by the way that the stove will actually stop running well before the canister pressure actually reaches one atmosphere, as some pressure is required to drive the gas through the jet and up the burner column where it drags in air to mix inside the burner.
Why is all this so? Why won't the presence of propane, which boils at -42 C, mean I can use the stove down to -40 C? First, as I explained above, any liquid will give off vapour below its boiling point: hot water cools by losing steam after all. But because the partial vapour pressure of the propane is a function of its percentage concentration, its contribution to the total vapour pressure (in the gas) will fall as the concentration in the liquid falls. In the case illustrated by the left hand graph we find that after about 40% of the contents has been used, the total vapour pressure inside the canister will be down to one atmosphere and the stove will die - even though there is still lots of butane and even some propane left inside. The right hand graph shows where the pressure crosses the one atmosphere line for different fuel mixes. Yes, the propane may be boiling, but it may not be making enough pressure all the same.
Pure propane maybe? Yes indeed, and very suitable. The problem is that on a hot day the propane vapour pressure may be more than one of these little canisters can handle. At 80 C, which could be reached under the rear window of a car on a very hot day, the VP of propane is 44 atmospheres or about 650 psi. That's why LPG (which is largely propane) comes in a heavy and more robust steel tank.
As I have hinted a couple of times above, the situation in the field is actually worse than that shown by the first graph. That graph assumes the temperature of the liquid stays at -18 C all the time. But it takes energy to vaporise the butane and propane, and that energy comes mainly from the liquid. It's the 'latent heat of vaporisation'. This means the liquid in the canister is going to get colder over time (unless you start pumping heat back into it). As the canister temperature falls, so the vapour pressures will also fall, as shown in the third graph (right hand one). It may be that the canister will stop releasing gas when only 20% of the contents have been used up. Of course, if you now heat the canister up the vapour pressures will rise and gas will come forth again. But if you are stuck at 3000 m and -18 C with no way of heating the canister, you may have a small problem.
To see what effect this has in the field, let's take the case from the first graph where the ambient is at -18 C: a very cold temperature for Australia. This is 17.5 C below the BP of butane, but only 6 C below the BP of isobutane. So the isobutane is, relatively speaking, much closer to its BP, and its vapour pressure will be much higher. If you are using an isobutane/propane mix your stove will keep working for a bit longer.
If we let the ambient be -5 C instead, the isobutane would be above its nominal BP, and your stove should have no trouble at all - until the gas in the canister cools down too far. So to skip all the maths, what it means is that an isobutane/propane canister is going to keep working for a lot longer in Australia, and under much colder conditions. But, of course, let it get cold enough and it too will fail.
OK, so which canisters are the good ones for cold weather? Using the right hand graph above we can see how the different gases behave. Let's take -5 C as a working temperature. A pure butane (eg Bluet puncture-style) canister would be below 1 atm, so it wouldn't work (obviously). A canister with 10% propane and 90% butane would be working, albeit poorly. The MSR pure isobutane would be a bit better, but not by very much. Adding just 10% propane to isobutane gives better results again, while adding 30 % propane to either butane or isobutane gives much better results. My interpretation of this is that the pure isobutane canisters, which seem to be dearer, may not be really worth the money: you might be better off using a standard 70% butane/30% propane canister and keeping it warm.
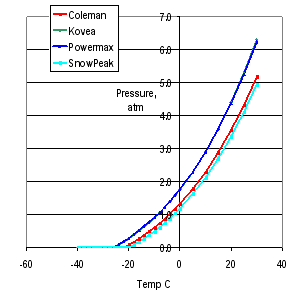
Now, what about the commercially available blends. In the graph to the right I have compared four of them: the Coleman, Kovea, Powermax and Snow Peak ones. The results are very interesting. The green curve for the Kovea 30% propane / 70% isobutane mix actually overlays the blue curve for the Coleman Powermax 40% propane / 60% butane. (Note: this is for the Powermax canister, not the common Coleman screw-thread 30% propane / 70 % butane one which is in red.) So while the 40% propane in the Powermax canister sounds really good, it is in fact no different from the 30% proipane in the Kovea. The Snow Peak 15% propane sounds poor, but in fact is little worse than the standard Coleman 30% propane because of the switch from butane to isobutane. Tricky stuff. Manufacturing decisions maybe?
Nonetheless, what is by now very clear is that using an upright canister stove at or below freezing can be risky. You must make sure that in cold climates you keep the canister warm so both gases come off at a decent pressure - or use a liquid feed stove.
I mentioned that the Coleman PowerMax and Fyrestorm stoves are very good in the cold. Why is this so? Why are they different from the others? The big reason lies in the use of a liquid feed rather than a gas feed. A liquid feed differs in two important ways.
If you use any of the other remote-tank gas stoves in the cold with the canister tipped upside down to provide a liquid feed, exactly the same comments will apply (but be careful). However, it still pays to keep the canister warm, as inspection of the graphs will show. A 70% butane / 30% propane mix will stay at that ratio to the very end, but even so at -15 C the pressure available to drive the stove is only 1/4 of an atmosphere (which is only a few psi for the older among us).
'Mixture' or 'solution': it doesn't matter. The actual boiling point will vary as the mixture inside the canister changes over time, as indeed it will in the field. Isobutane/propane mixes work better at low temperatures than the butane/propane mixes, and they work fine in the summer too. However, you are probably wise to avoid planning on taking an upright stove if you know the temperatures will be below freezing in the evenings. If you get caught, warm the canister inside your jacket first, and let some radiation from the stove hit the canister while you are cooking. Just make sure you can touch the canister at all times (see below).
If you are planning on a cold winter trip, and especially if you are planning on a snow trip, you should consider a liquid-feed stove. These work reliably quite a way down in temperature. However, below -20 C things do get difficult even for liquid-feed gas stoves, even with the best of fuel mixes. I thought this was below a reasonable range of temperatures until a Canadian gently explained to me that they often camped with obbvernight temperatures of -30 C. Now that is cold. To make matters worse, both the Powermax and the Kovea canisters hit zero gas flow around -24 C, so just getting the stove started might be very difficuult for said Canadian! That said, you can always warm up the canister just a little inside your jacket (or in your sleeping bag) to get it started, and then let some radiation from the stove hit the canister while you are cooking. Just make sure you can touch the canister at all times.
How can you tell how hot you can safely allow your canister to get? The following is just what I do: I cannot accept any resposibility for what you do. I put my hand on the canister. If it feels cold I prefer to let it warm up a little bit. If it feels cool to mildly warm, that's OK, but I keep an eye on it. If it starts to feel a bit 'hot' or above what I would call a comfortable hand temperature, I need to take some action to limit the thermal feedback: I may add the radiation shield. Actually, in warm weather I often add the radiation shield before I start cooking if I'm doing anything more than just make tea and coffee. If the canister feels too hot to touch comfortably I take action fast: I shut the stove down at once, and then start looking for the missing radiation shield. Typically, your hand can touch something below 45 C without it getting an 'ouch' response.
If you look at the last graph you can see that the pressures are starting to rising moderately fast above 20 - 30 C. I keep the canister below 'hand temperature', which is about 30 C. Warm is OK, 'hot' is not. However, note that the safety standards for these canisters require that they be able to handle significantly higher temperatures than this: think of inside a sealed car on a hot day for instance. My understanding is that all gas canisters must be able to take at least 50 C without any damage at all in order to pass the relevant European and American Standards. Some tests are run at even higher temperatures. The warnings the lawyers for some manufacturers make them put on the stoves about never using a windshield of any sort lest the canister explode are really way over the top! In fact, I regard those warnings as a serious safety hazard in their own right. Users find they can ignore that warning - and start to ignore all warnings. Not smart of the lawyers.
But there is a little more to watch here. If I notice the stove is starting to roar a bit, I always check the canister temperature. The roar suggests that the internal pressure is getting too high. You have been warned!
© Roger Caffin 22/4/2005