FAQ - Carbon Monoxide and Stoves
This article was summarised from "Backpacking Equipment Buyer's Guide", 1978
by William Kemsley & the editors of Backpacker Magazine
Copyright © 1978 by Backpacker, Inc
Reproduced with acknowledgment to Backpacker Magazine
Send any comments to the maintainer Roger Caffin
 |
Carbon Monoxide in Tents
Authors Joseph Kohler & Robert Wagner setting up a CO analyzer inside a tent to measure carbon monoxide dangers of stoves. The tent was made of breathable nylon but pitched without rain fly. During the tests, the tent's rear vent was open & the tent entrance was zipped partially open. After five minutes most stoves had filled the tent with dangerous quantities of carbon monoxide.
The authors wrote as follows:
|
While backpacking in New Hampshire's White Mountains last winter, four members of our party of 12 complained of dizziness and nausea after supper. The symptoms were the same as as those associated with altitude sickness, but we were camped at only 3400 feet. All four men felt fine the next morning, and the incident passed without explanation. Several months later we found a small winter-stove and cook-kit combination at a low price at a local hardware store. We bought a stove and decided to try it out by cooking lunch in an office at work. After 30 minutes, we noticed we felt dizzy and were experiencing mild headaches. We smelled combustion odors and decided to test for carbon monoxide. Our suspicions were confirmed when we found carbon monoxide levels of more than 100 parts per million (ppm) near the stove. We recalled the complaints of the four men during our winter trip. Unlike the rest of the group, they had cooked supper inside their tent because of severe winds and a low temperature. We decided their discomfort probably had resulted from exposure to a high level of carbon monoxide produced by their mountain stove.
Why Carbon Monoxide Is Dangerous.
Carbon monoxide (CO) is a colorless and almost odorless gas that results from the incomplete combustion of hydrocarbons. In the body some of the blood’s haemoglobin that ordinarily would pick up oxygen in the lungs picks up CO instead, so the amount of oxygen in the blood is reduced. The CO reacts with the haemoglobin to form carboxyhaemoglobin. The amount of carboxyhaemoglobin formed depends on the concentration of CO and the duration of exposure. Low amounts cause headache, dizziness and nausea; high amounts cause coma and death. An increase in altitude increases the severity of the effects because of the low oxygen saturation in one’s blood.
The Research
We decided to investigate the concentrations of CO produced in a tent by some common stoves. We used an uncoated nylon A-frame tent [in case it burnt down?]. We tested eight stoves: Bleuet (butane), Gerry Mini (liquid propane gas), Gerry Mini Mark II (1p gas), Primus Grasshopper (propane), Optimus 77A (methanol), Svea 123 (Coleman fuel), Optimus 111B (Coleman fuel) and MSR (Coleman fuel). The Svea 123 was tested with the Sigg Tourist Cook Kit, the Gerry Mini stoves in the Gerry Tourist Cookset and alone, and the Optimus 77A in the cookset sold with the stove, All stoves were clean and in excellent operating condition.
How the Stoves Were Tested.
An identical procedure was followed in all experiments. Stoves were centered 18 inches from the front entrance of the tent. Sampling probes were placed 28 inches from the center of the front entrance at a height of 24 inches, representative of the head level of a person cooking. Tubing connected the probes to two CO analyzers. Both analyzers were calibrated and checked against each other at levels below 100 ppm. Preliminary tests were conducted outdoors on a calm, 60 F day; extensive testing was done in a well-ventilated laboratory at 60 F. The tent was pitched normally, without its fly. The nine-inch circular vent in the rear was kept fully open, and the front flap was zipped partly closed to form a triangular opening measuring 10 x 10 x 12 inches at the tent’s apex. Clamps were placed on the zipper to ensure closing to the same position in each experiment. Prior to each experiment, the analyzers were zeroed and calibrated, and background levels of CO were recorded. They measured a maximum of 5 ppm at the beginning of each test and never exceeded 8 ppm.
Three pints of water at 60 F were placed in a 2½-quart aluminum pan (Sigg). Each stove was started outside the tent. As soon as it reached stable operating conditions it was placed inside the tent in the position indicated. The pot was centered on the burner, the door was quickly zipped shut as far as the clamp stops, and timing started. Concentrations of CO were recorded at one-half or one minute intervals until the levels stabilized, The stoves were run for 15 minutes.
What the Tests Showed.
The CO concentrations produced by the Optimus 77A, the Gerry Mini (with and without the Gerry Tourist Cookset), the Optimus 111B and the MSR rose rapidly during the first few minutes, then stabilized when the rate of CO escaping from the tent became equal to rate of CO produced by the stoves. The final concentrations, shown in the graph below, ranged from 70 to 130 ppm. (The Gerry Mini in the Tourist Cookset might have produced higher levels, but the stove ran out of fuel). The CO concentrations produced by the Bleuet, the Primus Grasshopper and the Svea 123 with the Sigg Tourist Cook Kit rose at a slower rate and stabilized at much lower levels – 20 to 25 ppm. The CO level produced by the Gerry Mini Mark II had not stabilized when the run was terminated.
What caused the high concentrations?
Clearly, the greater the rate of production of CO, the higher the final concentration in the tent. The rate of CO production depended on two factors: the amount of fuel burnt by the stove and the degree of incomplete combustion. The output was determined easily by by the output of energy: the Optimus 111B and MSR had high outputs; the Gerry Minis, the Optimus 77A and the Svea 123 had moderate outputs; and the Bleuet and Primus Grasshopper had lower outputs. Then we measured the degree of incomplete combustion by measuring the CO concentration very near each stove – by placing the probe from the ecolyzer close to the burner while the stove was operating without pans on its supports. Somewhat surprisingly we found that the Optimus 111B and 77A, MSR and Gerry Minis did not produce a CO concentration as high as that at head level inside the tent.
Then we repeated the tests for incomplete combustion, this time with pans of water placed on the stoves, and got quite different results. The concentrations of CO near the bottom of the pan were more than 200 ppm in every case except for the Svea 123 with the Sigg Cook Kit, which produced 30 ppm exactly as it had before when no pan was used. The only apparent reason for high production by the other stoves was that the flame impinged on the pan. We decided the cold pan surface caused quenching (cooling) of the flame, which resulted in incomplete combustion. The flame of the Svea 123 however did not impinge on the pan. [Added by RNC: one combustion path is from carbon to carbon monoxide to carbon dioxide: interrupting the process can leave the CO unburnt.]
We raised the pans on several stoves. When flames no longer impinged on the pans CO concentrations dropped. Next we modified several stoves to prevent flame impingement and lower the amount of CO produced. The Optimus 111B was modified by replacing the pan supports with supports 25 mm longer. The pan support for the MSR stove was first raised 18 mm, then 25 mm. The short windscreen on the Gerry Tourist Cookset was replaced by the windscreen from the Sigg Cook Kit, which was 30 mm taller. The original experiments were repeated using the modified stoves. The CO levels produced by both 'Gerry' Minis with the modified cookset and the modified Optimus 111B were dramatically reduced and were well within safe limits. The CO concentration produced by the MSR with the pot raised three-quarters of an inch was reduced, but still high; with the pot raised 25 mm the level was very low.
Conclusions
It is apparent from our tests that several of the high-output stoves produce high levels of carbon monoxide in a partially vented, breathable nylon tent as a result of incomplete combustion caused by flame quenching. It is likely that most other high-output stoves would produce similar levels and that even low-output stoves could produce high levels in a coated nylon tent. [Latter clause is doubtful if the low-output stoves are not producing much CO anyhow. RNC] Exposure to such levels of carbon monoxide probably is not dangerous at low altitudes although it may lead to discomfort. At altitudes above 10,000 feet, however, carbon monoxide poses a potentially dangerous hazard by reducing the already low oxygen saturation of the blood. The effects would depend also on the performance of the particular stove, the ventilation of the tent and the duration of cooking time.
Tables of Deadly Doses
Here's How Much Carbon Dioxide The Stoves Gave Off. The curves on the chart indicate incremental levels of carbon monoxide in the tent produced by the burning stoves. The Co measurements were taken at one minute intervals. The horizontal dotted line indicates the maximum level of carbon monoxide considered "safe" by the Amended Air Quality Act of 1970 [35 ppm].
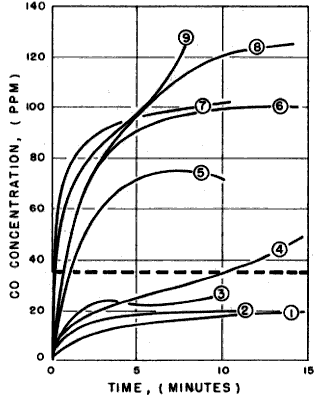 |
- Svea 123 in Sigg Tourist Cook Kit (Coleman fuel or petrol)
- Primus Grasshopper (propane)
- Bleuet (butane)
- Gerry Mini Mark II (lpg)
- MSR (Coleman fuel or petrol)
- Optimus 111B (Coleman fuel or petrol)
- Optimus 77A (metho)
- Gerry Mini (propane)
- Gerry Mini (propane) in Gerry Tourist Cookset
|
When Pot Supports Were Raised
The authors found that carbon monoxide dangers of stoves were reduced by raising stove pot supports 25 mm, high enough that the flame did not touch the pot bottom. While it took slightly longer to boil a pot of water, the carbon monoxide emission was lowered to safer levels.
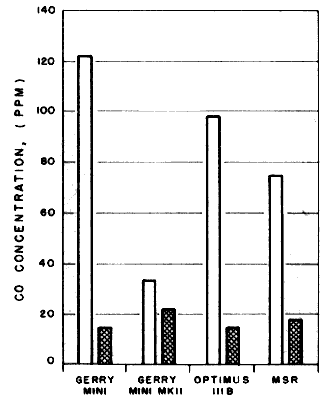 |
 |
Concentration of CO at boiling point, with pot supports raised 25 mm |
 |
Concentration of CO at boiling point, with stove as supplied by manufacturer |
|
Effects of Carbon Monoxide Poisoning
At sea level 97% of the haemoglobin in the blood is bound with oxygen as HbO2. However, carbon monoxide (CO) can combine with haemoglobin in the blood to form carboxyhaemoglobin (HbCO), and this reduces the ability of the blood to transport oxygen. The resulting condition is termed hypoxia. Hypoxia can also be caused by low oxygen pressure at high altitudes or by certain diseases. The amount of HbCO formed depends on the concentration of CO, the duration of exposure and the rate of breathing. The physiological effects of hypoxia increase progressively as the level of HbCO increases and the level of HbO2 decreases. The first symptoms appear at HbO2 levels between 95% and 92% saturation, when an individual may begin to experience improvement [sic, but probably should be 'degradation'.] in time interval discrimination, visual acuity and other psychomotor responses. The symptoms become more noticeable as the HbO2 level drops to 90% saturation, where the individual may experience drowsiness, lassitude and mental fatigue. At 85% saturation, headache, occasional nausea and euphoria may experienced. The symptoms intensify and are dominated by a throbbing headache as the concentration of HbO2 drops to 80% saturation. Vomiting and collapse occur at 70% saturation, coma at 60% and death at 40%. The ambient air quality standards as legislated by the 1970 Amended Air Quality Act limit the maximum average concentration of CO for a one-hour exposure to 35 ppm, or an HbO2 saturation level of 95.25%.
As an example, consider two winter mountaineers camped at a low elevation in an A-frame tent. Let’s say they spend two hours melting snow, boiling water and cooking, and that this exposes them to approximately 100 ppm CO from the stove. This would result in HbCO levels of 5%. The HbO2 level in their blood would be 92% (the normal 97% minus the 5% tied up as HbCO). The mountaineers might feel unpleasant and experience some impairment in visual acuity and other psychomotor responses but would be in no real danger.
However, the effects of breathing CO increase dramatically at higher altitudes. As altitude increases, atmospheric pressure decreases and oxygen saturation in the blood decreases. The following table shows the oxygen saturation at various altitudes for a person breathing pure air.
| Altitude (feet) |
Percent of 02 Saturation |
| 0 | 97% |
| 5,000 | 95% |
| 10,000 | 90% |
| 15,000 | 83% |
| 20,000 | 70% |
Since both altitude and CO reduce the oxygen saturation of the blood, their effects are approximately additive. Suppose our winter mountaineers cook in their tent at an elevation of 5,000 feet, instead. The HbO2 saturation in their blood would be 95% (the normal amount at 5,000 feet) minus the 5% reduction caused by the stove, or 90%. They would experience drowsiness, lassitude and mental fatigue. At 10,000 feet the mountaineers’ HbO2 levels would be 85% saturation. Headache, nausea and euphoria could ensue, and they would be in some danger. At altitudes higher than 10,000 feet, exposure to levels of CO becomes very dangerous. At altitudes of 17,000 feet, the mountaineers could vomit and collapse. These effects represent what typical mountaineers might experience under the conditions indicated, but they might vary considerably from one individual to another depending on physical condition, acclimatization to altitude and amount of exercise.


